
"People are living longer and therefore have more opportunity to contract shingles," he said.īut the rise is not linked to the vaccine. He noted that the United States does not keep national data on shingles doctors are not required to report cases of the condition.Ī few other things could explain the rise in shingles cases, Schaffner said.
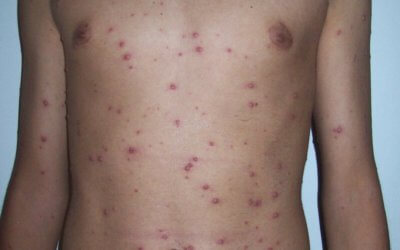
Since then, there has been at least a 90 percent drop in cases.īut while the number of cases of chickenpox has declined dramatically, the rate of shingles appears to be rising in areas where it has been studied, Schaffner said. 2000 Sep 13 284(10):1271-9.The CDC recommends that children receive two doses of the chickenpox vaccine, the first at age 12 months or 15 months, and the second between ages 4 years and 6 years.īefore the chickenpox vaccine was introduced in 1995, Schaffner said, about 4 million people in the United States, mostly children, got the disease each year. Postlicensure safety surveillance for varicella vaccine. Wise RP, Salive ME, Braun MM, Mootrey GT, Seward JF, et al. Prevention of Varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Safety of varicella vaccine after licensure in the United States: Experience from reports to the Vaccine Adverse Event Reporting System, 1995-2005. Update: Recommendations from the Advisory Committee on Immunization Practices (ACIP) Regarding Administration of Combination MMRV Vaccine. Klein NP, Yih WK, Marin M, Jumaan AO, Seward JF, et al. Herpes Zoster and Meningitis Resulting from Reactivation of Varicella Vaccine Virus in an Immunocompetent Child. Vaccine-associated Herpes Zoster Ophthalmicus and Encephalitis in an Immunocompetent Child. 2010 May 7 59(RR03):1-12.Ĭhouliaras G, Spoulou V, Quinlivan M, Breuer J, Theodoridou M. Use of Combination Measles, Mumps, Rubella, and Varicella Vaccine-Recommendations of the Advisory Committee on Immunization Practices. Marin M, Broder KR, Temte JL, Snider DE, Seward JF. Washington (DC): National Academis Press (US) 2011. Adverse Effects of Vaccines: Evidence of Causality. Stratton K, Ford A, Rusch E, Clayton EW, Committee to Review Adverse Effects of Vaccines Institute of Medicne, eds. Fatal Varicella Due to the Vaccine-Strain Varicella-Zoster Virus. Leung J, Siegel S, Jones JF, Schulte C, Blog D, Schmid DS, Bialek SR, Marin M. Closure of varicella-zoster virus-containing vaccines pregnancy registry – United States, 2013. Marin M, Willis ED, Marko A, Rasmussen SA, Bialek SR, et al. The profiles of serious and non-serious adverse events were consistent with previously published reports, and identified no new or unusual safety concerns. Researchers found a decrease of reported adverse events over time (around 500 per one million doses in 1995 and around 40 per one million doses in 2016) and serious adverse events comprised 0.8 reports per one million doses. The review included data collected from study reports submitted from March 17, 1995, through March 16, 2017, during which over 212 million doses were distributed globally.

Have a weakened immune system due to medication, history of hereditary or congenital immune system problems, or cancers, such as leukemia or lymphoma.Have had a severe, life-threatening allergic reaction after a previous dose of a chickenpox vaccine, or have any severe life-threatening allergies to a vaccine component, such as gelatin or neomycin.People should not get the chickenpox vaccine if they:


 0 kommentar(er)
0 kommentar(er)
